J Bradley Segal*
*Harvard Medical School, Boston, MA 02115, USA
Correspondence: JBradley_Segal@hms.harvard.edu
Synopsis
The average adult experiences two to five common colds each year. Summed up, people spend more than a year of life suffering from the illness. This article presents a brief report from an outbreak at Harvard Medical School followed by a review of what is currently known about the common cold. An emphasis is placed on illustrative experiments. Despite decades of research, hand washing remains the best method for preventing infection.
CASE STUDY
In August and September of 2014, there was an outbreak of an acute respiratory infection (ARI) among the first and second year students at Harvard Medical School and Harvard School of Dental Medicine. Out of 400 students, 74% (296) completed an anonymous retrospective survey concerning their recent health. Of the respondents, 34% of second year (57 of 167) and 25% of first year (33 of 129) students reported experiencing an acute illness over the preceding month. 94% (278 of 296) of the recently ill students reported experiencing one or several ARI symptoms, including nasal congestion, cough, sore throat, and nasal discharge. Incidence data were compiled from self-reported dates of when respondents first began feeling ill (Figure 1).
Figure 1. Incidence from Outbreak at Harvard Medical School. Among the 296 respondents to a retrospective survey, 90 students (30%) reported symptoms of an acute illness over a month-long period. 34% of second-year (57 of 167) and 25% of first-year (33 of 129) medical and dental students reported experiencing an acute illness over the previous month.
Behaviors Associated with Infection
The survey also asked respondents five questions concerning recent social behaviors. Relative risks of becoming ill were calculated for these dichotomous behavioral variables, both as a complete cohort and after stratifying the respondents based on class year (Table 1). Among both classes, the only two risk factors found to be significantly associated with becoming ill were recently going to a party or bar with classmates (RR 1.45, 95% CI 1.000 to 2.104, p = 0.0497) and frequently or always studying with classmates (RR 1.48, 95% CI 1.010 to 2.188, p = 0.0444). None of the other behaviors queried significantly altered the risk of contracting an acute illness.
Table 1. The Relative Risks of Contracting an Acute Illness from Various Dichotomous Behaviors
Data were calculated from anonymous surveys to medical and dental students, and relative risks calculated for first- and second-year classes, as well as in total. Among both classes, going to a bar or party with classmates in the last week and studying with classmates some or all of the time significantly increased the risk of contracting the illness. For both classes, living in the medical school dorm, spending more than 30 min a day in the medical education building, or regularly attending lecture did not significantly alter the risk of becoming ill.
Infectiousness
Kermack and McKendrick’s compartmental epidemiological model was used to calculate the basic reproduction number R0 for the outbreak under the assumption that ill medical students were infected by their classmates (Kermack and McKendrick, 1991). For the combined classes, R0 was calculated to be 3.5, indicating that the average ill student infected 3.5 of his or her classmates. By way of comparison, estimated R0 for other infectious diseases include 2.7 for the 1918 A/H1N1 influenza pandemic (Mills et al., 2004), 1.5 for the 2009 H1N1 pandemic (Yang et al., 2009; Fraser et al, 2009), 3.6 for the 2003 SARS outbreak (Wallinga and Teunis, 2004), and 1.73–2.02 for the 2014 Ebola virus epidemic (WHO Ebola Response Team, 2014).
Behavioral Model of Disease Transmission
The probability of illness transmission is proportional to the product of the number of contact events sufficient for transmission between individuals and the per-event likelihood of transmission. Kermack and McKendrick’s compartmental model treats populations as homogenous and combines these two factors into a single parameter that represents the transmission rate across the population. This approach is useful for large populations where following individual interactions are impractical or when no information on social network structure is available. While this model can predict the total number of individuals who will be ill at a given time, it neither gives any information on how illnesses are dispersed among subsets of the population norprovides the opportunity to use behavioral data to predict transmission.
This study had both a small population and limited behavioral data. These allowed for the construction of a discrete-time Markov model of disease transmission through the class, wherein probability of a transmission event between any pair of students was proportional to the behavioral similarity of the pair. Ideally, such a behavioral model has the advantages of allowing a better prediction of the spread of disease prospectively and an understanding of the specific social interactions that promote disease transmission retrospectively. Unfortunately, in the current study, the model informed by social behavior did not predict the spread of illness any better than Kermack and McKendrick’s compartmental model. This is likely because the behavioral survey lacked the granularity to adequately capture pairwise social interactions. The behavioral variance calculated from survey responses was small across the population, and students who reported similar behaviors may not have preferentially interacted with one another. In future studies, capturing data more descriptive of pairwise interactions—for example, via construction of a social network wherein network distances are taken to be the probability of interaction sufficient for transmission—may allow this method to be used to build an informative model of disease spread in a small population.
Other Considerations
The magnitude of this outbreak was larger than would be expected based on prior studies of ARI’s among small communities in relative isolation (Warshauer et al., 1989; Flynn et al., 1977). The identification of a pathogen is not required to diagnose the common cold, but it is possible that the pathogens responsible for this outbreak were heterogeneous in nature (Heikkinen and Jarvinen, 2003). While informal studies such as this may intuitively seem as if they can inform medical students who wish to avoid catching a cold during the school year, classic and contemporary research has unraveled more profound insights into common cold pathogenesis, transmission, and prevention
INTRODUCTION
How Common is the Cold?
Cohorts of medical students have or likely will experience occasions when a mysterious ARI rapidly sweeps through their flu-vaccinated class. The common cold is a mild ARI characterized by some combination of malaise, rhinorrhea (nasal discharge), nasal congestion, headache, cough, sneezing, sore throat, and low-grade fever (Jackson et al., 1958). While the cold is unfortunately considered “low yield” for USMLE Step 1 purposes, respiratory infections are the most common cause of illness in industrialized countries (Denny, 1995) and are likely the most common cause of illness worldwide (Papadopoulos, 1999). Adults have two to five colds per year, totaling in a lifetime to over a year spent with the disease (Papadopoulos, 1999; Johnston et al., 1996). Twenty-five million patients in the US visit the doctor with an ARI chief complaint every year, resulting in $726 million spent on unnecessary antibiotic prescriptions (Gonzales et al., 2001). One study found that 76% of elderly patients with viral common colds were prescribed antibiotics (Nicholson et al., 1996). The 500 million domestic cases of non-influenza ARI’s directly cost the US healthcare system $17 billion annually (Fendrick et al., 2003). By comparison, influenza has a direct medical cost of $10.4 billion annually (Molinari et al., 2007). The cold is a significant source of lost productivity as well (Molinari et al., 2007), causing adults in the US to miss 20 million days of work annually (Adams et al., 1996), with indirect costs of $22.5 billion (Fendrick et al., 2003).
Relevance to Doctors in Training
Given the absence of effective treatments or means of diagnosis, the common cold remains pertinent to medical students because unintentionally transmitting an ARI to any of several vulnerable patient populations with whom medical students interact can significantly raise a patient’s risk of death (Meibalane et al., 1977; Strausbaugh et al., 2003; Malavaud et al., 2001; Horcajada et al., 2003; Dolan et al., 2012). Infection with the cold can cause asthma and chronic obstructive pulmonary disease (COPD) exacerbation, frequently leading to hospitalization (Nicholson et al., 1993; Mallia et al., 2011; Teichtahl et al., 1997). For immunocompromised individuals, the cold can mean serious complications and possibly death (Ghosh et al., 1999). Even among elderly patients, a cold lasts twice as long, has more severe symptoms, and has double the risk of a lower airway complication such as pneumonia (Nicholson et al., 1996, 1997).
Despite the prevalence and economic impact of the disease, the common cold is not proportionally emphasized in medical education. An understanding of the cold is needed to refrain from prescribing patient’s unindicted antibiotics, and, in lieu of effective treatments, medical providers should know the proven preventative measures. By understanding the fundamental features of common cold transmission, medical students can significantly lower the chances that they spread the infection (Jefferson et al., 2011). Such an understanding will contextualize asthmatic and COPD patients who present in the emergency room short of breath following an otherwise-harmless cold (Teichtahl et al., 1997). By learning about the cold, a prudent student can also minimize his or her own productivity lost to illness as well. Finally, insights into mankind’s most common infection can help one understand and contextualize more malicious infectious diseases.
CAUSES
Viral Distribution
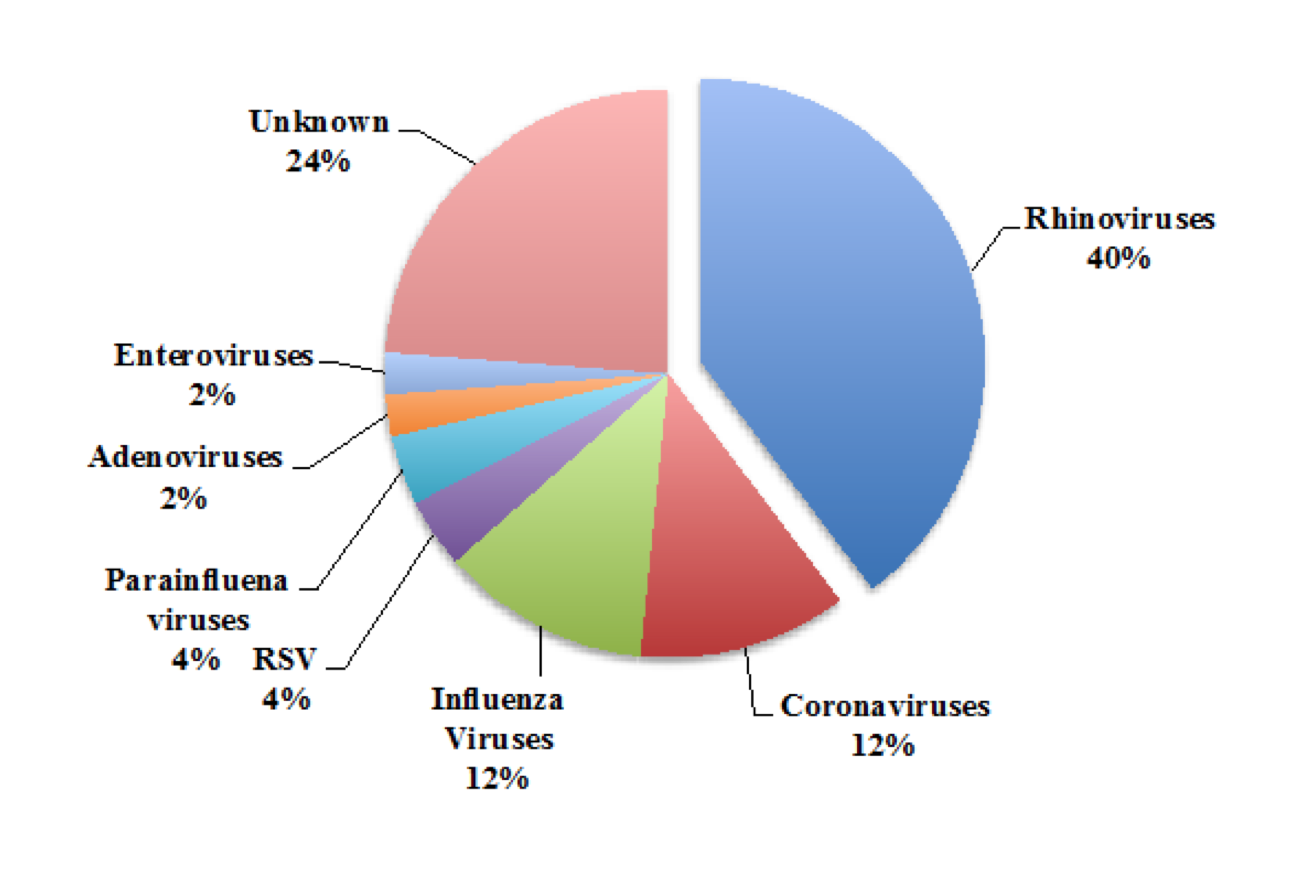
Figure 2. The Proportion of Common Cold Cases Caused by Each Type of Virus. The cold is caused by a diverse arrangement of viruses. Approximately one out of four common colds have an unknown cause, and there are likely still undiscovered viral pathogens (van den Hoogen et al., 2001). The numbers shown above change throughout the year as most of the viruses associated with the common cold display seasonality. For example in the autumn, the 100 serotypes of the rhinovirus can cause up to 80% of common colds (Arruda et al., 1997). Data adapted from Heikkinen and Jarvinen (2003).
The common cold does not have a single cause. Rather, the cold is caused by a host of viruses with strikingly diverse phylogenetics (Figure 2). Across all age groups, the most common cause of the cold is the rhinovirus, accounting for around half of common cold infections (Monto and Sullivan, 1993). The rhinovirus displays season-dependent transmission, and during its peak in autumn, the pathogen causes up to 80% of colds (Arrudaet al., 1997). Together the coronaviruses, respiratory syncytial viruses (RSVs), and parainfluenza viruses, adenoviruses and enteroviruses account for around 35% of colds (Fendrick et al., 2003). Influenza viruses cause around 5%–15% of colds. Because the common cold is defined on the basis of its clinical presentation, a mild influenza infection can accurately be diagnosed as a cold, meaning that the two infections are not completely distinct disease entities (Heikkinen and Jarvinen, 2003). It is suspected that yet-unidentified viruses explain the remaining 20%–30% of the cases of the cold. For example human metapneumovirus has a worldwide distribution but was only discovered in children with the cold in 2001 (van den Hoogen et al., 2001).
Rhinovirus and ICAM-1
As rhinovirus is the most frequent cause of the common cold (Monto and Sullivan, 1993), the pathogen will be the primary focus of this review The rhinovirus infects epithelial cells of the nasopharynx. Viral particles gain access to the epithelium by the mouth or nose or from eyes via the lacrimal duct (Hendley, 1999). The eyes and nose are the most common routes of inoculation (Hendley et al., 1973). It is known neither how the rhinovirus gains direct access to cells within the nasal mucosa nor if the rhinovirus can infiltrate an intact mucosal membrane (Winther, 2011). 90% of rhinovirus serotypes enter epithelial cells in the nasopharynx after binding the surface protein ICAM-1 (Greve et al., 1989). This receptor is selectively expressed by certain epithelial cells, with a high concentration among non-ciliated epithelial cells of the nasopharyngeal tonsil (adenoid) (Teichtahl et al., 1997). Successful rhinovirus infection leads to an upregulation of ICAM-1 and downregulation of an endogenous decoy ICAM-1, thereby enhancing the viruses’ infectivity (Whiteman et al., 2003). Rhinovirus can spread from a simple ARI and infect epithelial cells in the lower airway as well (Papadopoulos et al., 2000). Subsets of epithelial cells in the lower airway also express ICAM-1, though at a lower density than in the upper airway (Mosser et al., 2002). The optimal temperature for rhinovirus replication is 33°C–35°C (Hayden, 2004). In healthy adults, the nasopharynx temperature is usually 34°C (Keck et al., 2000). Despite being deeper in the body, areas of the lower respiratory tract fall within rhinoviruses’ replication range as well. For example, the carina is 33.2°C during normal breathing (Hayden, 2004). Among infants, rhinovirus is the second most common cause of pneumonia and bronchiolitis, largely due to its ability to infect the lower airway (Hayden, 2004).
ICAM-1, Asthma, and Clinical Symptoms
Epithelial ICAM-1 expression is upregulated following inflammation and mediates subsequent neutrophil migration (Vejlsgaard et al., 1989; Smith et al., 1988). As asthma is a disease characterized in part by bronchial inflammation, patients with asthma tend to have basally elevated ICAM-1 expression levels in the lower airways (Wegner et al., 1990). This potentially explains the strong association between cold infections and acute asthma exacerbations. Rhinovirus infections in asthma patients are known to cause morbidity and sometimes mortality (Johnston et al., 1996). It is estimated that between 50%–80% of asthmatic exacerbations are caused by the cold (Johnston et al., 1995, 1996). One study found that 37% of patients who required hospitalization for an acute asthma attack had a viral ARI (Teichtahl et al., 1997). Hospital admission is for asthma patients the strongest predictor of 12-month mortality (Crane et al., 1992). A laboratory infection of 13 non-asthmatic volunteers with COPD showed that rhinovirus infection leads to COPD exacerbation and lower respiratory symptoms, though the role of ICAM-1 in these patients is less clear (Mallia et al., 2011).
Interestingly, 25% of patients infected with a cold-associated virus remain clinically asymptomatic (Gwaltney and Hayden, 1992). Adults are more likely to remain asymptomatic during an infection than children (Peltola et al., 2008). Children also have more severe colds. Among children, 70% have colds that last at least 10 days (Pappas et al., 2008), as opposed to only 20% of adults (Gwaltney et al., 1967). It has been proposed that acquired immunity and variations in ICAM-1 expression with age may explain why some individuals have active viral infections but remain asymptomatic (Peltola et al., 2008). One study found that polymorphisms of ICAM-1 were associated with varying susceptibility to common cold illnesses (Nieters et al., 2001). However, common cold cases in this study were self-reported, and it is unclear if individuals with “protective” ICAM-1 genotypes were more likely to resist initial infection of epithelial cells or if infected individuals were more likely to remain asymptomatic. Still, ICAM-1 remains a promising target for future research aimed at preventing rhinovirus infection.
SYMPTOMS
Clinical Presentation
Because of the variation in clinical symptoms that patients with a common cold experience, it has not been possible to develop a pathognomic characterization of the disease (Eccles, 2005). Diagnosis is made clinically from reported symptoms with good reliability (Heikkinen and Jarvinen, 2003). Nine out of ten patients who diagnose themselves with the cold are found to have an identifiable virus (Arruda et al., 1997). Experimentally, there are eight classic symptoms of the cold: sneezing, malaise, headache, chilliness, nasal discharge, nasal obstruction, cough, and sore throat (Jackson et al., 1958). Not all of these are present in every patient with a cold, and a physical exam may sometimes reveal conjunctiva injection (bloodshot eyes) and pharyngeal erythema.
Time Course of Symptom Progression
Figure 3. Clinical Symptoms of the Cold Tend to Change over the Course of the Illness. The symptoms of the cold tend to present and resolve in a predictable pattern. Often an ill-defined headache and malaise are the first symptoms patients notice. Most patients experience a sore throat by day 2 post-exposure. This gives way to nasal discharge and nasal obstruction. A cough is usually the last symptom to appear and often persists past the resolution of the other symptoms to around day 7 to 10 post-exposure (data not shown; Heikkinen and Jarvinen, 2003). Diagnostically this information has limited utility because the exact clinical course differs for every patient (Kirkpatrick, 1996). Results adapted from Jackson et al. (1958).
Clinical symptoms tend to occur at overlapping but consistent time points during the course of an illness (Figure 3). Though incubation period depends to a large extent on the type of virus causing the cold (Bradburne et al., 1967), patients usually begin experiencing their first symptoms 24–72 hr after exposure (Heikkinen and Jarvinen, 2003). Classically, patients experience a sore throat 1 to 2 days after exposure, and the percent of patients experiencing a sore throat quickly dissipates after day 2 (Tyrrell et al., 1993). Patients then experience nasal discharge and obstruction between days 2 and 5, which gives way to cough by about day 6 post-exposure (Jackson et al., 1958; Tyrrell et al., 1993). On average, symptoms in healthy adults tend to spontaneously resolve after 7–10 days, with the cough generally being the last symptom to resolve (Heikkinen and Jarvinen, 2003).
In contrast, symptoms in elderly patients can take twice as long to resolve (median 16 days), and the risk of lower airway involvement is doubled (Nicholson et al., 1996). Children have the cold for longer as well, with most cases lasting at least 10 days (Pappas et al., 2008). Children also tend to have slightly different symptomatic progression than adults. One study found 88% of children with the cold experienced nasal congestion and 75% nasal discharge on day 3 of illness—which is similar but more prevalent than in adults—but with cough peaking earlier on day 2 and remaining in half of children through day 8 of illness (Pappas et al., 2008).
Pathogen Identification
When narrowing down a differential diagnosis, the cold can be distinguished from similar illnesses on a clinical basis. Simple rhinitis will not present with a sore throat or cough, and bacterial tonsillitis will not present with a runny nose or nasal obstruction. The cold rarely presents with a high fever, the presence of which along with cold-like symptoms is suggestive of the flu. During periods of high flu activity, the CDC recommends that patients with this clinical presentation be rapidly triaged to minimize potential influenza exposure to healthcare workers and other patients. Pertussis may initially present as a common cold would, but coughing will persist for more than 2 weeks, and there may also be apnea or vomiting present.
Both the common cold and acute bacterial rhinosinusitis can present with purulent nasal discharge (thick, colored) (Wald et al., 1991). Sputum color is indicative of an inflammatory response but not of any specific pathogen (Eccles, 2005). Hence when clinically assessing an ARI, sputum color is a poor prognostic tool for determining whether antibiotics ought to be prescribed (Murray et al., 2000). Antibiotic treatment is indicated when a clinical diagnosis of acute bacterial rhinosinusitis is made on the basis of severe maxillary pain in the face or teeth, particularly if the pain is unilateral, and fever, or rhinosinusitis symptoms and maxillary pain lasting more than 7 days (Hickner et al., 2001). However the majority of acute rhinosinusitis cases that last fewer than 7 days will resolve spontaneously, and antibiotics ought to be withheld (Hickner et al., 2001).
While the cold-associated viruses can be individually identified using PCR assays, because the infection is typically benign and self-limiting, such identification is not medically indicated. Each family of viruses has slight variations in its presentation and pathogenesis. For example, one study found that 40% of patients with PCR-confirmed rhinovirus infections initially presented with a sore throat, but only 25% of rhinovirus-negative patients had this initial presentation (Arruda et al., 1997). However, accurately differentiating the common cold-associated viruses on a clinical basis alone is not possible (Nicholson et al., 1997; Arruda et al., 1997; Kirkpatrick, 1996).
PATHOGENESIS
Role of the Immune Response
The rhinoviruses do not directly cause observable damage to host tissue (Winther et al., 1984a, 1986). The only observable change under histology is an increased number of polymorphonuclear leukocytes in the nasal mucosa following infection (Winther et al., 1984b). Because epithelial cells are left unscathed, it is believed that cold symptoms are caused completely or to a significant extent by an immune response and are not a direct result of viral pathogenesis (Hendley, 1999). The immune response, mediated by signaling molecules released directly or indirection from infected epithelial cells, results in bradykinin release, which is associated with increased vascular permeability of the venous sinuses, thereby causing the cold’s hallmark symptoms of nasal discharge and congestion (Proud et al., 1990). Applying bradykinin in the noses of healthy volunteers mimics these symptoms of the cold (Proud et al., 1988) and occurs in a dose-dependent fashion (Doyle et al., 1990). A handful of other pro-inflammatory cytokines and chemokines explain the other common cold symptoms such as sneezing, headache, fever, and malaise (Kirchberger et al., 2007). The culpability of the immune response in causing the common cold symptoms have earned it the nickname the, “cytokine disease” (Kirchberger et al., 2007).
Because the inflammatory response to the cold causes infected epithelial cells to undergo apoptosis and subsequent extrusion, it has been proposed that the immune response limits local viral spread (Winther, 2011). Hence, a therapeutic intervention that restricts the immune response for the purposes of symptom suppression may theoretically exacerbate an infection or prolong viral shedding (http://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-pathogenesis-of-rhinovirus-infections). However, this remains incompletely understood—for unknown reasons, application of nitric oxide both tapers the immune response to a cold and results in faster rhinovirus clearance (Proud, 2005). This is an area of research that may yield new therapeutic targets for common cold treatment.
The Cold in Immunocompromised Patients
Studies of the common cold among patients with impaired immune systems allude to the complexity of the immune system’s role in rhinovirus infection. In one study of 22 severely myelosuppressed, immunocompromised adults who contracted rhinovirus, 33% of the patients developed a fatal pneumonia at an average of 12 days after the onset of cold symptoms (Ghosh et al., 1999). A more recent study found similar rates of rhinovirus pneumonia among immunosuppressed patients (Jacobs et al., 2013). While 60% of these patients also had a bacterial, viral or fungal co-infection, rhinovirus was the sole detectable pathogen in 40% of the patients with pneumonia (Jacobs et al., 2013). In a study of the first 100 days after hematopoietic cell transplantation, immunosuppressed patients did become symptomatic following rhinovirus or coronavirus infection (Milano et al., 2010). Of these, around 15% of patients continued to shed virus for 3 months or more, but 13% of patients had detectable viral particles and never reported developing clinical symptoms. Another small prospective surveillance study following hematopoietic cell transplantation found that pediatric patients with a rhinovirus infection were more likely to remain asymptomatic and shed viral particles then develop clinical symptoms (Srinivasan et al., 2013). Two patients in this study asymptomatically shed rhinovirus for 14 and 34 days before developing symptoms. Another study found that among immunosuppressed adult lung transplant recipients, higher rhinovirus titer was associated with clinical symptoms of the cold, while patients with lower viral loads had clinical symptoms far less frequently (Gerna et al., 2009).
There are limited data on common cold infections among immunocompromised individuals, but the available studies demonstrate that an intact immune system is required to minimize the risk of morbidity and mortality from the cold. Interestingly, immunocompromised patients can still show the signs and symptoms characteristic of an immune response to a cold infection. These patients may not be any more likely to remain asymptomatic than healthy adults with active rhinovirus infections (Gwaltney and Hayden, 1992; Peltola et al., 2008), but in some immunocompromised individuals there is a prolonged latent asymptotic phase before the patients mount an immune response. It can also take months for these patients to successfully extinguish a cold infection.
TRANSMISSION
Hand Contact and Fomites
The classic means by which the cold is transmitted is self-inoculation from a healthy individual’s own fingertips (Hendley et al., 1973). Usually a person will contaminate his or her fingers and spread the virus by touching his or her own eyes or nose, which gives the virus access to the nasal mucosa (see “Causes” above). Cold viruses find their way onto hands from either direct contact with someone actively shedding the virus—such as a handshake—or indirectly from contact with an infected environmental surface. A study using PCR in hotel rooms found that ill individuals shed viral particles on 33%–60% of commonly touched fomites like door handles, TV remotes, and light switches (Winther et al., 2007). Rhinoviruses can survive on environmental surfaces for several hours (Gwaltney et al., 1982). Even though viral titer drops by an order of magnitude when a droplet containing active virus dries out, viral traces that are undetectable via tissue culture can still cause an infection (Winther, 2011).
One study in a pediatric ward found that wearing plastic goggles that covered the eyes and nose when holding infants with an ARI decreased infections by 43% among infants and 34% among healthcare workers (Gala et al., 1986). Another study found that 0 of 14 adult participants seated near but physically separated from infants with RSVs became ill, 4 of 10 adults who touched the infants became ill, and 5 of 7 adults who held and played with the infants fell ill (Hall et al., 1981). These results reinforce the conclusion that when caring for someone with the cold, preventing self-inoculation from hand-eye or hand-nose contact is essential for avoiding infection of oneself and others.
Saliva
Saliva is a poor conduit for viral transmission—90% of people with a cold have undetectable levels of virus in their mouth (Kirkpatrick, 1996). Infection that results from kissing a person with the cold is considered a rare occurrence, likely because of the low titer of virus in saliva and because inoculation of pharyngeal mucosa rarely causes infection (Hendley et al., 1973; D’Alessio et al., 1976).
Aerosolized Droplets
Infections caused by aerosolized droplets have been documented, but this is not considered a significant route of transmission (Winther, 2011). Many studies have demonstrated that touching a person with the cold leads to infection far more frequently than physical proximity to a sick individual alone. It has been proposed that perhaps the recirculation of air may increase the chances of cold transmission by raising the risk of exposure to aerosolized droplets. To test this hypothesis, researchers compared the incidence of colds 1-week post-airplane flight for 1,100 travelers. Half the passengers flew on planes that recirculated cabin air and the other half flew on planes with fresh air ventilation. The researchers found the recirculation of air in the context of commercial flights had no significant effect on cold transmission (Zitter et al., 2002). However, it should be noted that this finding is at odds with data from military barracks, where living in a closed-ventilation barracks was found to raise the relative risk of catching a ARI by 1.5 (95% CI 1.46 – 1.56) (Brundage et al., 1988).
Viral Shedding and Contagiousness
Viral shedding peaks 48–72 hr after infection (Hendley and Gwaltney, 2004). In one study, 24 married couples were monitored after a spouse was inoculated with rhinovirus. The researchers found that the risk factors for successful pathogen transmission were high viral load, moderate symptoms, and time spent with spouse (D’Alessio et al., 1976). This finding indicated that viral spread depends on peak viral load coinciding with the presence of only mild symptoms. Hence, most transmissions of the cold tend to occur within the first 5 days after exposure. Even though symptoms usually taper off after 5–7 days, viral shedding continues for up to 2 weeks after infection, meaning a recently ill person can still unknowingly spread the cold (Winther et al., 1986).
PREVENTION
Vaccination
Several distinct families of viruses cause the cold (Monto and Sullivan, 1993). Even among the most common cause of the cold, the rhinovirus, there are over 100 virus serotypes, thus far thwarting vaccination efforts (Heikkinen and Jarvinen, 2003). Targeted therapies might still be possible, since many pathogeneses share common pathways. For example, upregulating a decoy form of ICAM-1—the surface protein that 90% of rhinoviruses use to gain entry into epithelial cells—decreases rhinovirus infectivity in vitro (Whiteman et al., 2003). The recent discovery of conserved motifs among broad serotypes of rhinovirus may also potentially yield targets for drug development (Poland and Barry, 2009; Palmenberg et al., 2009). Non-vaccine strategies have been the primary area of research in cold prevention research.
Pharmaceutical Prophylaxis
Importation into a household by school-aged children is a common route of cold transmission to adults (Monto and Sullivan, 1993). Hence, one preventative strategy is to take aggressive measures that stop the infection of family members when one member of a household catches the cold.
Prophylactic pharmaceutical agents demonstrated to prevent cold infections exist, but these still carry unpalatable side effects. For example in one study, whenever a participant developed a cold, his or her family would begin a 7-day prophylactic course of intranasal interferon (Douglas et al., 1986). The treatment reduced ARI illnesses among family members by 41%, but each course of treatment had around a 12% risk of intranasal bleeding. Intranasal interferon was particularly efficacious for rhinovirus infections, decreasing infections by 86%. However, it is not possible to clinically determine if a cold is caused by rhinovirus as opposed to another viral pathogen, limiting the utility of the observed efficacy (Nicholson et al., 1997 ;Arruda et al., 1997; Kirkpatrick, 1996). Additionally, interferon use led to a concerning leukocyte accumulation in the mucosa (Hayden et al., 1987). Other antiviral chemotherapies (e.g., ICAM-1 blocker, capsid binding agents, and protease inhibitors) have similarly failed to show a promising risk to benefit ratio (Winther, 2011).
Weather and Isolation
Contrary to popular belief, there is no demonstrated association between being in cold weather and common cold susceptibility. Newcomers and long-time workers at a remote research base in Antarctica were found to be equally susceptible to catching the cold (Warshauer et al., 1989). While a medical school class’ isolation might seem protective, another Antarctica study of ARI’s during a period of absolute isolation found that a respiratory infection present at the beginning of isolation persisted throughout the 6 months of winter isolation (Flynn et al., 1977).
Vitamin C
Another popular means of cold prevention, vitamin C supplementation, was examined in a meta-analysis of 29 trials (Hemila and Chalker, 2013). Together the studies include 10,708 participants from the general public and show vitamin C supplementation does not decrease common cold incidence, either when taken regularly or in large prophylactic doses (RR 0.97, 95% CI 0.94 to 1.00) (Hemila and Chalker, 2013). Because vitamin C does not reduce cold incidence in the general public, the authors suggested that routine vitamin C supplementation “is not justified.” While regular supplementation of vitamin C was associated with an 8% reduction of symptom duration in adults and 14% reduction in children, given the wide variation of cold presentations (Monto and Sullivan, 1993), it is possible that this statistical finding is entirely sub-clinical and is not relevant for a population-wide recommendation. Additionally, it was found that mega-doses of vitamin C after the onset of clinical symptoms had no effect on illness duration or symptom intensity.
Curiously, five studies of 598 extreme athletes under conditions of intense but brief physical stress—individuals at a ski camp (Ritzel, 1961), runners in an ultra-marathon (Peters et al., 1993), etc.—receive a clear and consistently positive benefit from vitamin C supplementation (Hemila and Chalker, 2013). For example, in one study half of the Canadian military recruits taking part in arctic training exercises were given a daily placebo and the other half were given daily vitamin C (Sabiston and Radomski, 1974). To minimize bias, the supplemented group was only revealed to both participants and researchers at the end of the study through the measurement of intravenous ascorbate levels. Among 112 men, 25% taking the placebo and only 10% receiving vitamin C caught the cold. The five studies show that vitamin C supplementation under conditions of acute physical stress cuts the incidence of cold infections by half (RR 0.48, 95% CI 0.35 to 0.64) (Hemila and Chalker, 2013). Two other randomized controlled trials found that individuals under conditions of prolonged physical stress—marine recruits at boot camp and competitive adolescent swimmers—received no benefit from vitamin C in terms of cold prevention (Pitt and Costrini, 1979; Constantini et al., 2011). This perhaps indicates that the supplementation with vitamin C has a substantial benefit, but only under conditions of acute physical stress (Hemila and Chalker, 2013).
Hence, aside for a narrow subset of extreme athletes, vitamin C has no demonstrated therapeutic benefit. Yet, even if it will not help treat or prevent the cold, especially given the low risk and cost of vitamin C, supplementation does not hurt.
Surgical Masks
Social NetworksWhile masks can prevent cold transmission in hospital wards—especially when they prevent self-inoculation by covering the eyes and nose (Gala et al., 1986)—surgical masks have not yet been demonstrated to be effective in more general contexts. One prospective study found that wearing a surgical mask has no effect on likelihood of catching a cold, but it did significantly make a mask-wearing participant more likely to experience headaches (Jacobs et al., 2009). Yet it is worth noting that this 77-day study with 32 participants was underpowered and would only have detected an absolute risk reduction of 60% from wearing a mask. Unfortunately, other drastic or novel approaches have not yet shown great promise either (Jefferson et al., 2011). For example, use of tissue paper with virucidal properties did not effectively reduce the frequency of colds (Farr et al., 1988).
Figure 4. Early Infection of Central Individuals in an Outbreak. Your friends tend to be more centrally located in social networks than you are. Hence in the conditions of social spreading, “central” individuals in a social network pick up infections earlier than random individuals. Researchers were able to use this feature of social networks in real-time to detect an influenza outbreak significantly earlier than traditional surveillance methods (Christakis and Fowler, 2010). Theoretical results adapted from Christakis and Fowler (2010).
Contemporary studies into the social component of disease transmission have utilized quirky features of social networks to successfully prevent the spread of infectious diseases and computer viruses. For example, the targeted vaccination of “central” individuals who have the most connections in a social network can raise population immunity (Pastor-Satorras and Vespignani, 2002; Cohen et al., 2003). Because central individuals have more connections in a social network, they are likely to spread a disease to more people and become infected earlier in an outbreak. Another feature of social networks is the so-called “friendship paradox,” which is the observation that your friends have more friends than you do—or that your friends are likely more central than you are. Researchers used this during a flu outbreak at Harvard College and found that if random volunteers nominated a friend, because that friend was more likely to be a central individual—and hence more likely to get sick earlier in an outbreak—monitoring the nominated, central friends for signs of the flu significantly improved early flu detection (Figure 4) (Christakis and Fowler, 2010). At this time, however, a study utilizing social network structure for early detection or prevention has not been attempted for the common cold.
Hand Washing — The Punch Line
What do we have to prevent the cold, then? The answer can be gleaned from a classic experiment in 1980 in which one group of random volunteers dipped their fingers in dilute iodine solution—it was known to have virucidal properties (Hendley et al., 1978)—and were compared to volunteers who dipped their hands into water that was died to look and smell like iodine (Gwaltney et al., 1980). Immediately after drying their hands, volunteers made hand contact with rhinovirus-positive donors who had just picked their noses (“The donors contaminated their hands with nasal secretions by finger-to-nose contact”), and 15 min later, volunteers touched their own eyes and noses. This was repeated for 3 days. None of the eight iodine-exposed volunteers became infected, while all seven controls became ill (p < 0.001). Unfortunately, routine iodine use is impractical given that many patients do like having iodine-stained hands.
Subsequent randomized controlled trials demonstrated that good hand hygiene leads to a 20% decrease in cold incidence (Carabin et al., 1999; Ladegaard and Stage, 1999). One crossover study found that giving children hand-sanitizer to compliment normal hand washing resulted in a 50% decline in ARI incidence (Dyer et al., 2000). A meta-analysis of 67 studies on preventing ARI transmission concurred hygienic measures are the most effective measure to prevent ARI infection (Jefferson et al., 2011).
CONCLUSION
As discussed throughout this review, many of the most illustrative studies of the common cold rely on despotic study protocols. Some studies leveraged drastic geographical conditions—at Antarctic research bases and military exercises on the Northern frontier—to study the cold in isolation (Warshauer et al., 1989; Flynn et al., 1977; Sabiston and Radomski, 1974). Other researchers intentionally infected healthy volunteers with the cold, ranging from the infection of married people to study risk factors associated with transmission (D’Alessio et al., 1976) to infecting COPD patients simply to prove that the cold causes COPD exacerbation (Mallia et al., 2011). Such measures were not utilized for the purposes of the brief case study of the outbreak at Harvard Medical School. Good-hearted volunteers and the self-limited nature of the cold have made it possible for researchers to illuminate the pathogenesis, transmissions, and prevention of mankind’s most common ailment.
The case study from Harvard Medical School revealed two risk factors significantly associated with contracting an ARI. However, the gross social patterns of behavior elucidated in the study did not capture unique social interactions with the granularity needed to prospectively predict the spread of disease or retrospectively describe the specific social interactions that tended to promote disease transmission. Disease transmission in social networks is an unexplored area of research in common cold transmission, and the methods discussed above have implications for preventing and detecting outbreaks among small semi-isolated communities such as universities, hospital wards, military bases, and retirement communities. Such prevention efforts are especially important given that in this outbreak ill students each infected 3.5 of their colleagues.
Thus far, good hand hygiene is the best method of preventing common cold transmission, especially when around children (Jefferson et al., 2011). A survey of the literature supports this intuitive conclusion—self-inoculation from one’s fingers through the eyes or nose is the most frequent means by which the cold is transmitted (Hendley et al., 1973). Saliva and aerosolized droplets rarely cause infections (Winther, 2011; Kirkpatrick, 1996; D’Alessio et al., 1976). Yet infectious viral particles can persist on hands and commonly used fomites for hours (Winther et al., 2007; Gwaltney et al., 1982). Peak viral shedding coincides with early cold symptoms such as rhinorrhea (Jackson et al., 1958; Hendley and Gwaltney, 2004). Cold symptoms are completely attributable to our immune response (Hendley, 1999). Children tend to have more colds per year than adults (Pappas et al., 2008) and are frequently responsible for exposing family members to the numerous cold pathogens (Monto and Sullivan, 1993). Annually, the cold accrues more direct medical costs than influenza and is the number one reason for missed work and school (Fendrick et al., 2003; Molinari et al., 2007). There are no effective treatments for the cold (http://www.uptodate.com/contents/the-common-cold-in-adults-treatment-and-prevention). This makes prevention especially important for vulnerable populations such as asthmatic, COPD, elderly, and immunocompromised patients (Nicholson et al., 1996; Mallia et al., 2011; Teichtahl et al., 1997; Ghosh et al., 1999). Alas, as a prevention strategy, hand washing is almost disappointingly simple. But given hand washing’s safety (consider nose bleeds from intranasal interferon) (Douglas et al., 1986; Hayden et al., 1987), ease (consider surgical masks in public) (Jacobs et al., 2009), and efficacy (consider vitamin C) (Hemila and Chalker, 2013), perhaps a simple solution is not a bad thing for such a common problem.
Acknowledgments
The author would like to thank Annie Morgan for essential help with data analysis for the case study at Harvard Medical School and extends another warm thank you to the HMSR staff for their patience with this manuscript. The author has no conflicts of interest to disclose.
References
Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, (1996). Vital and health statistics Series 10. Data from the National Health Survey 1999, 1–203.
Arruda, E., Pitkaranta, A., Witek, T.J., Jr., Doyle, C.A., and Hayden, F.G. (1997). Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 35, 2864–2868.
Sabiston, B.H., and Radomski, M.W. (1974). Health Problems and Vitamin C in Canadian Northern Military Operations. Defence and Civil Institute of Environmental Medicine Report 74-R-1012.
Bradburne, A.F., Bynoe, M.L., and Tyrrell, D.A. (1967). Effects of a “new” human respiratory virus in volunteers. BMJ 3, 767–769.
Brundage, J.F., Scott, R.M., Lednar, W.M., Smith, D.W., and Miller, R.N. (1988). Building-associated risk of febrile acute respiratory diseases in Army trainees. JAMA 259, 2108–2112.
Carabin, H., Gyorkos, T.W., Soto, J.C., Joseph, L., Payment, P., and Collet, J.P. (1999). Effectiveness of a training program in reducing infections in toddlers attending day care centers. Epidemiology 10, 219–227.
Christakis, N.A., and Fowler, J.H. (2010). Social network sensors for early detection of contagious outbreaks. PLoS ONE 5, e12948.
Cohen, R., Havlin, S., and Ben-Avraham, D. (2003). Efficient immunization strategies for computer networks and populations. Phys. Rev. Lett. 91, 247901.
Constantini, N.W., Dubnov-Raz, G., Eyal, B.B., Berry, E.M., Cohen, A.H., and Hemila, H. (2011). The effect of vitamin C on upper respiratory infections in adolescent swimmers: a randomized trial. Eur. J. Pediatr. 170, 59–63.
Crane, J., Pearce, N., Burgess, C., Woodman, K., Robson, B., and Beasley, R. (1992). Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int. J. Epidemiol. 21, 737–744.
D’Alessio, D.J., Peterson, J.A., Dick, C.R., and Dick, E.C. (1976). Transmission of experimental rhinovirus colds in volunteer married couples. J. Infect. Dis. 133, 28–36.
Denny, F.W., Jr. (1995). The clinical impact of human respiratory virus infections. Am. J. Respir. Crit. Care Med. 152, S4–S12.
Dolan, G.P., Harris, R.C., Clarkson, M., et al. (2012). Vaccination of health care workers to protect patients at increased risk for acute respiratory disease. Emerg. Infect. Dis. 18, 1225–1234.
Douglas, R.M., Moore, B.W., Miles, H.B., et al. (1986). Prophylactic efficacy of intranasal alpha 2-interferon against rhinovirus infections in the family setting. N. Engl. J. Med. 314, 65–70.
Doyle, W.J., Boehm, S., and Skoner, D.P. (1990). Physiologic responses to intranasal dose-response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J. Allergy Clin. Immunol. 86, 924–935.
Dyer, D.L., Shinder, A., and Shinder, F. (2000). Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism. Fam. Med. 32, 633–638.
Eccles, R. (2005). Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 5, 718–725.
Farr, B.M., Hendley, J.O., Kaiser, D.L., and Gwaltney, J.M. (1988). Two randomized controlled trials of virucidal nasal tissues in the prevention of natural upper respiratory infections. Am. J. Epidemiol. 128, 1162–1172.
Fendrick, A.M., Monto, A.S., Nightengale, B., and Sarnes, M. (2003). The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 163, 487–494.
Fraser, C., Donnelly, C.A., Cauchemez, S., et al. (2009). Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324, 1557–1561.
Gala, C.L., Hall, C.B., Schnabel, K.C., et al. (1986). The use of eye-nose goggles to control nosocomial respiratory syncytial virus infection. JAMA 256, 2706–2708.
Gerna, G., Piralla, A., Rovida, F., et al. (2009). Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J. Med. Virol. 81, 1498–1507.
Ghosh, S., Champlin, R., Couch, R., et al. (1999). Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 29, 528–532.
Gonzales, R., Malone, D.C., Maselli, J.H., and Sande, M.A (2001). Excessive antibiotic use for acute respiratory infections in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 33, 757–762.
Greve, J.M., Davis, G., Meyer, A.M., et al. (1989). The major human rhinovirus receptor is ICAM-1. Cell 56, 839–847.
Gwaltney, J.M., Jr., and Hayden, F.G. (1992). Psychological stress and the common cold. N. Engl. J. Med. 326, 644–645, author reply 5–6.
Gwaltney, J.M., Jr., and Hendley, J.O. (1982). Transmission of experimental rhinovirus infection by contaminated surfaces. Am. J. Epidemiol. 116, 828–833.
Gwaltney, J.M., Jr., Hendley, J.O., Simon, G., and Jordan, W.S., Jr. (1967). Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA 202, 494–500.
Gwaltney, J.M., Jr., Moskalski, P.B., and Hendley, J.O. (1980). Interruption of experimental rhinovirus transmission. J. Infect. Dis. 142, 811–815.
Hall, C.B., and Douglas, R.G., Jr. (1981). Modes of transmission of respiratory syncytial virus. J. Pediatr. 99, 100–103.
Hayden, F.G. (2004). Rhinovirus and the lower respiratory tract. Rev. Med. Virol. 14, 17–31.
Hayden, F.G., Winther, B., Donowitz, G.R., Mills, S.E., and Innes, D.J. (1987). Human nasal mucosal responses to topically applied recombinant leukocyte A interferon. J. Infect. Dis. 156, 64–72.
Heikkinen, T., and Jarvinen, A. (2003). The common cold. Lancet 361, 51–59.
Hemila, H., and Chalker, E. (2013). Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 1, Cd000980.
Hendley, J.O. (1999). Clinical virology of rhinoviruses. Adv. Virus Res. 54, 453–466.
Hendley, J.O., and Gwaltney, J.M., Jr. (2004). Viral titers in nasal lining fluid compared to viral titers in nasal washes during experimental rhinovirus infection. J. Clin. Virol. 30, 326–328.
Hendley, J.O., Wenzel, R.P., and Gwaltney, J.M., Jr. (1973). Transmission of rhinovirus colds by self-inoculation. N. Engl. J. Med. 288, 1361–1364.
Hendley, J.O., Mika, L.A., and Gwaltney, J.M., Jr. (1978). Evaluation of virucidal compounds for inactivation of rhinovirus on hands. Antimicrob. Agents Chemother. 14, 690–694.
Hickner, J.M., Bartlett, J.G., Besser, R.E., Gonzales, R., Hoffman, J.R., and Sande, M.A. (2001). Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann. Emerg. Med. 37, 703–710.
Horcajada, J.P., Pumarola, T., Martinez, J.A., et al. (2003). A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur. Respir. J. 21, 303–307.
Jackson, G.G., Dowling, H.F., Spiesman, I.G., and Boand, A.V. (1958). Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch. Intern. Med. 101, 267–278.
Jacobs, J.L., Ohde, S., Takahashi, O., Tokuda, Y., Omata, F., and Fukui, T. (2009). Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am. J. Infect. Control 37, 417–419.
Jacobs, S.E., Soave, R., Shore, T.B., et al. (2013). Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 15, 474–486.
Jefferson, T., Del Mar, C.B., Dooley, L., et al. (2011). Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. Cd006207.
Johnston, S.L., Pattemore, P.K., Sanderson, G., et al. (1995). Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310, 1225–1229.
Johnston, S.L., Pattemore, P.K., Sanderson, G., et al. (1996). The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am. J. Respir. Crit. Care Med. 154, 654–660.
Keck, T., Leiacker, R., Riechelmann, H., and Rettinger, G. (2000). Temperature profile in the nasal cavity. Laryngoscope 110, 651–654.
Kermack, W.O., and McKendrick, A.G. (1991). Contributions to the mathematical theory of epidemics–I. 1927. Bull. Math. Biol. 53, 33–55.
Kirchberger, S., Majdic, O., and Stockl, J. (2007). Modulation of the immune system by human rhinoviruses. Int. Arch. Allergy Immunol. 142, 1–10.
Kirkpatrick, G.L. (1996). The common cold. Prim. Care 23, 657–675.
Ladegaard, M.B., and Stage, V. (1999). Ugeskr. Laeger 161, 4396–4400.
Malavaud, S., Malavaud, B., Sandres, K., et al. (2001). Nosocomial outbreak of influenza virus A (H3N2) infection in a solid organ transplant department. Transplantation 72, 535–537.
Mallia, P., Message, S.D., Gielen, V., et al. (2011). Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Respir. Crit. Care Med. 183, 734–742.
Meibalane, R., Sedmak, G.V., Sasidharan, P., Garg, P., and Grausz, J.P. (1977). Outbreak of influenza in a neonatal intensive care unit. J. Pediatr. 91, 974–976.
Milano, F., Campbell, A.P., Guthrie, K.A., et al. (2010). Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 115, 2088–2094.
Mills, C.E., Robins, J.M., and Lipsitch, M. (2004). Transmissibility of 1918 pandemic influenza. Nature 432, 904–906.
Molinari, N.A., Ortega-Sanchez, I.R., Messonnier, M.L., et al. (2007). The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25, 5086–5096.
Monto, A.S., and Sullivan, K.M. (1993). Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol. Infect. 110, 145–160.
Mosser, A.G., Brockman-Schneider, R., Amineva, S., et al. (2002). Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J. Infect. Dis. 185, 734–743.
Murray, S., Del Mar, C., and O’Rourke, P. (2000). Predictors of an antibiotic prescription by GPs for respiratory tract infections: a pilot. Fam. Pract. 17, 386–388.
Nicholson, K.G., Kent, J., and Ireland, D.C. (1993). Respiratory viruses and exacerbations of asthma in adults. BMJ 307, 982–986.
Nicholson, K.G., Kent, J., Hammersley, V., and Cancio, E. (1996). Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ 313, 1119–1123.
Nicholson, K.G., Kent, J., Hammersley, V., and Cancio, E. (1997). Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 315, 1060–1064.
Nieters, A., Brems, S., and Becker, N. (2001). Cross-sectional study on cytokine polymorphisms, cytokine production after T-cell stimulation and clinical parameters in a random sample of a German population. Hum. Genet. 108, 241–248.
Palmenberg, A.C., Spiro, D., Kuzmickas, R., et al. (2009). Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324, 55–59.
Papadopoulos, N.G. (1999). JSRIZA, Banatvala J, Pattison J. Principles and practice of clinical virology, Fourth Edition (Chichester, UK: John Wiley & Sons).
Papadopoulos, N.G., Bates, P.J., Bardin, P.G., et al. (2000). Rhinoviruses infect the lower airways. J. Infect. Dis. 181, 1875–1884.
Pappas, D.E., Hendley, J.O., Hayden, F.G., and Winther, B. (2008). Symptom profile of common colds in school-aged children. Pediatr. Infect. Dis. J. 27, 8–11.
Pastor-Satorras, R., and Vespignani, A. (2002). Immunization of complex networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 65, 036104.
Peltola, V., Waris, M., Osterback, R., Susi, P., Ruuskanen, O., and Hyypia, T. (2008). Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J. Infect. Dis. 197, 382–389.
Peters, E.M., Goetzsche, J.M., Grobbelaar, B., and Noakes, T.D. (1993). Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am. J. Clin. Nutr. 57, 170–174.
Pitt, H.A., and Costrini, A.M. (1979). Vitamin C prophylaxis in marine recruits. JAMA 241, 908–911.
Poland, G.A., and Barry, M.A. (2009). Common cold, uncommon variation. N. Engl. J. Med. 360, 2245–2246.
Proud, D. (2005). Nitric oxide and the common cold. Curr. Opin. Allergy Clin. Immunol. 5, 37–42.
Proud, D., Reynolds, C.J., Lacapra, S., Kagey-Sobotka, A., Lichtenstein, L.M., and Naclerio, R.M. (1988). Nasal provocation with bradykinin induces symptoms of rhinitis and a sore throat. Am. Rev. Respir. Dis. 137, 613–616.
Proud, D., Naclerio, R.M., Gwaltney, J.M., and Hendley, J.O. (1990). Kinins are generated in nasal secretions during natural rhinovirus colds. J. Infect. Dis. 161, 120–123.
Ritzel, G. (1961). Helv. Med. Acta 28, 63–68.
Smith, C.W., Rothlein, R., Hughes, B.J., et al. (1988). Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J. Clin. Invest. 82, 1746–1756.
Srinivasan, A., Flynn, P., Gu, Z., et al. (2013). Detection of respiratory viruses in asymptomatic children undergoing allogeneic hematopoietic cell transplantation. Pediatr. Blood Cancer 60, 149–151.
Strausbaugh, L.J., Sukumar, S.R., and Joseph, C.L. (2003). Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin. Infect. Dis. 36, 870–876.
Teichtahl, H., Buckmaster, N., and Pertnikovs, E. (1997). The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest 112, 591–596.
Flynn, T.C., Fusch, L.W., and Dick, E.C. (1977). Colds and immunity in the 77 winter personnel at McMurdo Station and Scott Base, 1976. Antartic Journal 1977;October:5-6.
Tyrrell, D.A., Cohen, S., and Schlarb, J.E. (1993). Signs and symptoms in common colds. Epidemiol. Infect. 111, 143–156.
van den Hoogen, B.G., de Jong, J.C., Groen, J., et al. (2001). A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7, 719–724.
Vejlsgaard, G.L., Ralfkiaer, E., Avnstorp, C., Czajkowski, M., Marlin, S.D., and Rothlein, R. (1989). Kinetics and characterization of intercellular adhesion molecule-1 (ICAM-1) expression on keratinocytes in various inflammatory skin lesions and malignant cutaneous lymphomas. J. Am. Acad. Dermatol. 20, 782–790.
Wald, E.R. (1991). Purulent nasal discharge. Pediatr. Infect. Dis. J. 10, 329–333.
Wallinga, J., and Teunis, P. (2004). Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am. J. Epidemiol. 160, 509–516.
Warshauer, D.M., Dick, E.C., Mandel, A.D., Flynn, T.C., and Jerde, R.S. (1989). Rhinovirus infections in an isolated antarctic station. Transmission of the viruses and susceptibility of the population. Am. J. Epidemiol. 129, 319–340.
Wegner, C.D., Gundel, R.H., Reilly, P., Haynes, N., Letts, L.G., and Rothlein, R. (1990). Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science 247, 456–459.
Whiteman, S.C., Bianco, A., Knight, R.A., and Spiteri, M.A. (2003). Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J. Biol. Chem. 278, 11954–11961.
WHO Ebola Response Team (2014). Ebola virus disease in West Africa–the first 9 months of the epidemic and forward projections. N. Engl. J. Med. 371, 1481–1495.
Winther, B. (2011). Rhinovirus infections in the upper airway. Proc. Am. Thorac. Soc. 8, 79–89.
Winther, B., Brofeldt, S., Christensen, B., and Mygind, N. (1984a). Light and scanning electron microscopy of nasal biopsy material from patients with naturally acquired common colds. Acta Otolaryngol. 97, 309–318.
Winther, B., Farr, B., Turner, R.B., Hendley, J.O., Gwaltney, J.M., Jr., and Mygind, N. (1984b). Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol. Suppl. 413, 19–24.
Winther, B., Gwaltney, J.M., Jr., Mygind, N., Turner, R.B., and Hendley, J.O. (1986). Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA 256, 1763–1767.
Winther, B., McCue, K., Ashe, K., Rubino, J.R., and Hendley, J.O. (2007). Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J. Med. Virol. 79, 1606–1610.
Yang, Y., Sugimoto, J.D., Halloran, M.E., et al. (2009). The transmissibility and control of pandemic influenza A (H1N1) virus. Science 326, 729–733.
Zitter, J.N., Mazonson, P.D., Miller, D.P., Hulley, S.B., and Balmes, J.R. (2002). Aircraft cabin air recirculation and symptoms of the common cold. JAMA 288, 483–486.